Scientific Integrity in the Federal Agencies: An Unfinished Agenda
Dedicated scientific integrity policies in the federal agencies have been under development for over a decade. In 2007, enactment of the America COMPETES Act included a provision that required that the Office of Science and Technology Policy (OSTP) and the Office of Management and Budget (OMB) oversee “develop and issue an overarching set of principles to ensure the communication and open exchange of data and results to other agencies, policymakers, and the public of research conducted by a scientist employed by a Federal civilian agency and to prevent the intentional or unintentional suppression or distortion of such research findings.” These principles were further expected to be codified within federal agencies as “specific policies and procedures” (P.L. 110-69, Section 1009).
Under the Obama administration implementation of these scientific integrity policies got off to a fast start, but soon languished and were never fully implemented across all agencies. Under the Trump Administration, despite the provisions of the 2007 America COMPETES Act, the development and implementation of agency scientific integrity policies have been ignored, if not completely abandoned or undermined. This post reviews some history and context of the federal government’s unfinished scientific integrity agenda.
In March 2009, soon after his inauguration, President Obama issued a memorandum requesting that within 120 days that OSTP coordinate and oversee the development of scientific integrity policies across the federal agencies. In December, 2010 OSTP director John Holdren issued a follow-up memo with further guidance for federal agencies in developing scientific integrity policies. Subsequently, at least 24 departments and agencies of the federal government developed scientific integrity policies including (most relevant to the COVID-19 pandemic) the Department of Health and Human Services, the National Institutes of Health, the Centers for Disease Control, and the Food and Drug Administration.
Here is some of the language from those policies in public health agencies:
HHS: “HHS shall sustain a culture of scientific integrity. Scientific progress depends upon honest investigation, open discussion reflecting a balance of diverse scientific views, refined understanding, and a firm commitment to evidence. Science, and public trust in science, thrives in an environment that shields scientific data and analyses from inappropriate political influence. Political officials should not suppress or alter, nor appear to suppress or alter, scientific or technological findings.”
NIH: “Scientific integrity, in this context, refers to maintaining the quality and objectivity of the research activities that the National Institutes of Health (NIH) funds and conducts, such that they are sound and worthy of the public’s confidence. NIH’s commitment to sound, objective science also strengthens the public’s trust in policy decisions informed by scientific data.”
CDC: “CDC has a responsibility to conduct the best science and is committed to disseminating scientific findings and results without being influenced by policy or political issues. Although CDC may conduct research in areas relevant for making policy decisions, the goal of such research is to provide the best evidence to drive policy in the right direction.”
FDA: “A culture of scientific integrity is one that ensures that scientific decisions are the product of honest investigation, open discussion, refined understanding, and a firm commitment to evidence, and at the same time are shielded from inappropriate political influence.”
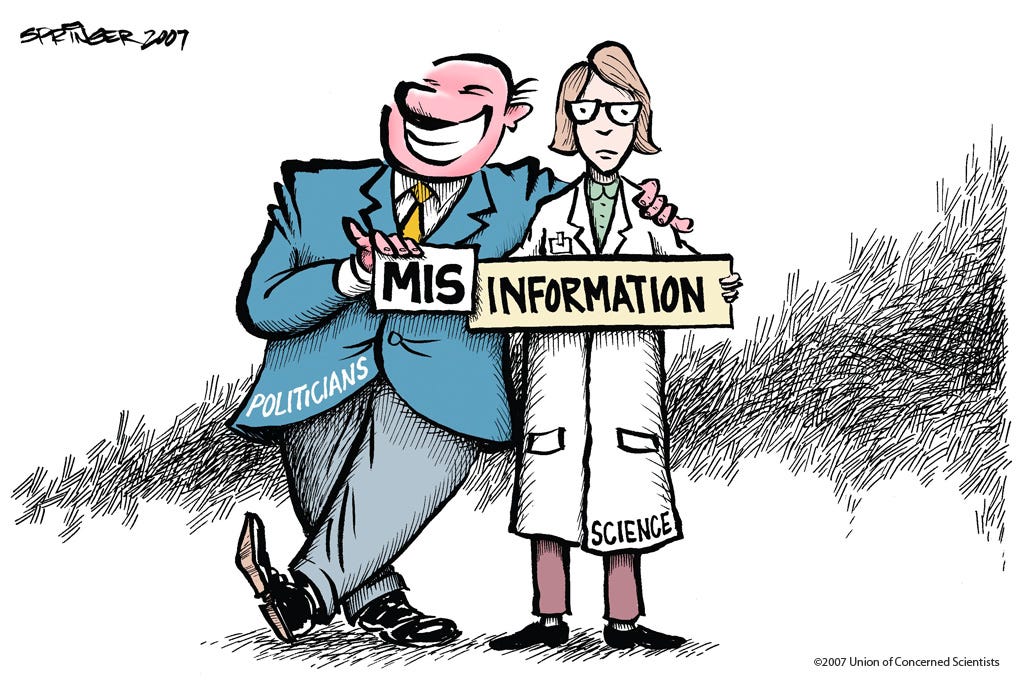
As is well-documented (see, for instance, this post and links therein), under the Trump Administration these agencies have not always lived up to following the principles expressed in their scientific integrity policies. In fact, in some cases — most notably CDC — the Trump Administration has arguably decimated scientific integrity.
The scientific integrity failures under the Trump Administration indicates the insufficiency of administrative action alone, such as that requested under the America COMPETES act and initiated under President Obama. To better ensure scientific integrity in federal agencies, Congress needs to act to elevate principles and guidance into federal law subject to Congressional oversight and judicial review.
In 2011, the Obama Administration took its foot off the gas in its push to implement scientific integrity policies in the agencies. There is an important lesson here.
One reason for this sudden loss of interest is that the 2010 election resulted in a transition from a Democratic to Republican majority in the House of Representatives. Scientific integrity guidelines empower majority opposition parties (to the president) in Congress to conduct more vigorous oversight of science, evidence and data in executive branch policy making. The Obama administration was not about to hand Republicans in the House enhanced tools of oversight, even for something as important as scientific integrity. So divided government meant that progress on scientific integrity stalled.
And of course, in 2017 the Trump administration came in with no interest in scientific integrity (or perhaps even science), divided government or not. Under the current policy architecture for scientific integrity, in the absence of executive branch interest, scientific integrity policies may languish. In the context of executive branch ineptitude or malevolence, scientific integrity with federal agencies may even be compromised.
Evidence suggests that even in the presence of such policies, implementation can be uneven. In 2016, a report commissioned by OSTP to evaluate implementation of Obama administration scientific integrity policies found a wide range of inconsistent agency actions, even including inconsistencies in how “scientific integrity” was being defined. Similarly, a 2019 GAO report looked at 9 agencies and found that while scientific integrity policies continued to exist (in 2018), agencies displayed considerable variability in their implementation.
In the event of the election of Joe Biden along with Democratic control of both houses of Congress there may open a window of opportunity for scientific integrity legislation to be enacted. The failures of scientific integrity in the pandemic show why it is so badly needed. Congress has a start on this legislation (see, e.g., H.R 1709 from the current Congress), but more thought and attention is needed to address the consequences of scientific integrity failures of the Trump Administration.
Ultimately, well-conceived scientific integrity legislation will constrain members of both parties from the pathological politicization of science in policy and politics, and that is something we should all get behind, no matter who we vote for.